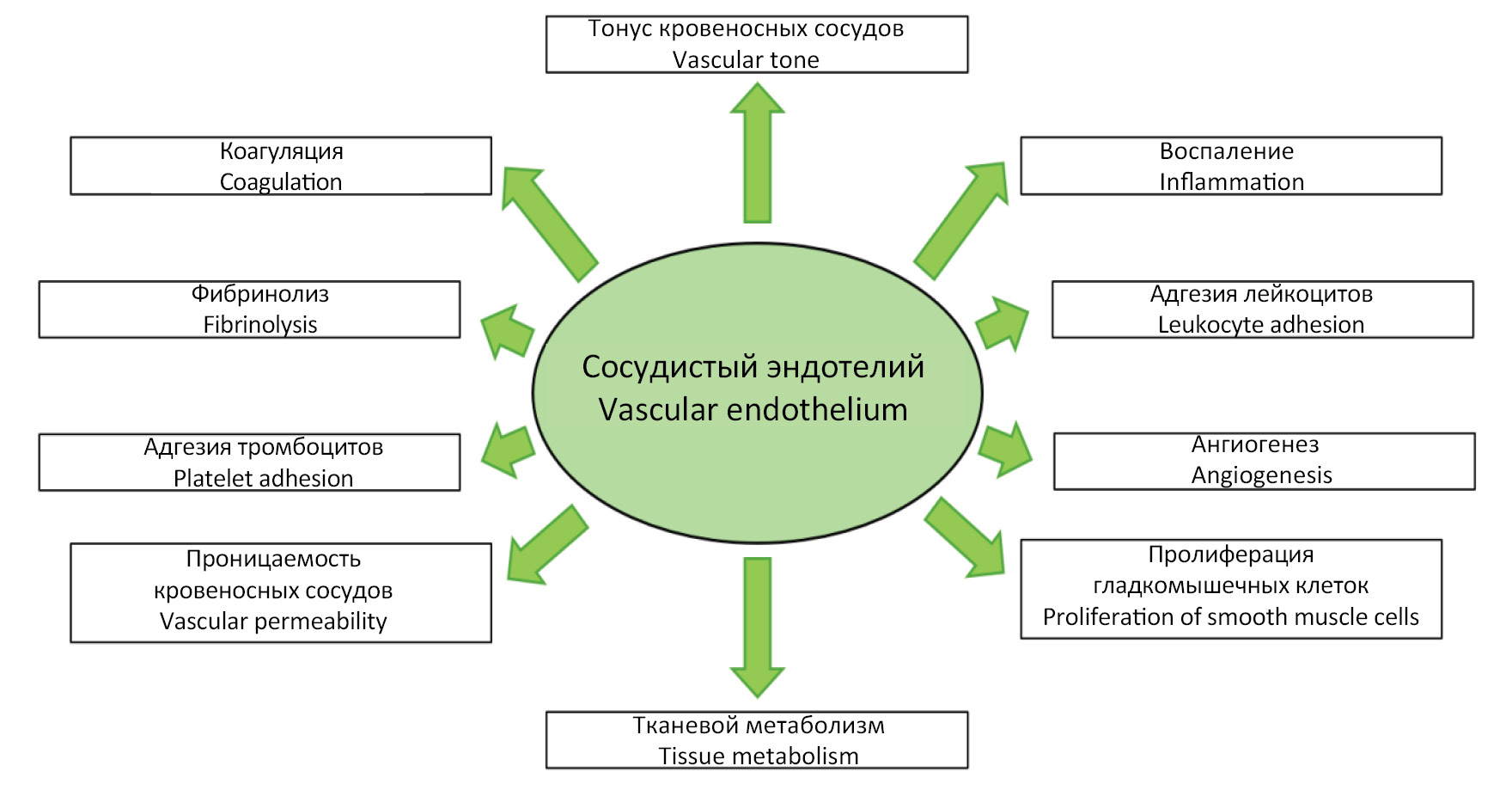
Endothelial activation and dysfunction caused by influenza A virus (Alphainfluenzavirus influenzae)
- Authors: Marchenko V.A.1, Zhilinskaya I.N.1
-
Affiliations:
- North-Western State Medical University Named after I.I. Mechnikov
- Issue: Vol 69, No 6 (2024)
- Pages: 465-478
- Section: REVIEWS
- URL: https://ogarev-online.ru/0507-4088/article/view/277909
- DOI: https://doi.org/10.36233/0507-4088-264
- EDN: https://elibrary.ru/zujoza
- ID: 277909
Cite item
Abstract
Annual epidemics of influenza result in 3–5 million cases of severe illness and more than 600 000 deaths. Severe forms of influenza are usually characterized by vascular endothelial cells damage. Thus, influenza A viruses, including subtypes A(H1N1)pdm09, A(H3N2), as well as highly pathogenic avian influenza viruses, can infect the vascular endothelium, leading to activation and subsequent dysfunction of these cells. In turn, endothelial dysfunction resulting in systemic morphofunctional changes of endothelial cells, which leads to impaired vascular tone, thrombosis and other complications, and is also a risk factor and profoundly implicated in the pathogenesis of many cardiovascular diseases. Thus, endothelial dysfunction is an important aspect of the pathogenesis of severe influenza, which must be considered in the pathogenetic therapy of this infectious disease.
The aim of the review is to analyze the causes and specify mechanisms of development of endothelial activation and dysfunction caused by influenza A virus.
Full Text
##article.viewOnOriginalSite##About the authors
Vladimir A. Marchenko
North-Western State Medical University Named after I.I. Mechnikov
Author for correspondence.
Email: vmarcenco@mail.ru
ORCID iD: 0000-0001-6870-3157
Ph. D. in medicine, Associate Professor of Medical Microbiology Department
Russian Federation, 191015, St. PetersburgIrina N. Zhilinskaya
North-Western State Medical University Named after I.I. Mechnikov
Email: vmarcenco@mail.ru
ORCID iD: 0000-0002-0084-1323
D. Sc. in Biology, Professor of Medical Microbiology Department
Russian Federation, 191015, St. PetersburgReferences
- Office WHOEMR. Global Influenza Strategy 2019–2030. Weekly Epidemiological Record; 2019.
- Boehme A.K., Luna J., Kulick E.R., Kamel H., Elkind M.S.V. Influenza-like illness as a trigger for ischemic stroke. Ann. Clin. Transl. Neurol. 2018; 5(4): 45663. https://doi.org/10.1002/acn3.545
- Muscente F., De Caterina R. Causal relationship between influenza infection and risk of acute myocardial infarction: pathophysiological hypothesis and clinical implications. Eur. Heart J. 2020; 22(Suppl. E): E68–72. https://doi.org/10.1093/eurheartj/suaa064
- Skaarup K.G., Modin D., Nielsen L., Jensen J.U.S., Biering-Sørensen T. Influenza and cardiovascular disease pathophysiology: strings attached. Eur. Heart J. 2023;25(Suppl. A): A5–11. https://doi.org/10.1093/eurheartjsupp/suac117
- Rubino R., Imburgia C., Bonura S., Trizzino M., Iaria C., Cascio A. Thromboembolic events in patients with influenza: a scoping review. Viruses. 2022; 14(12): 2817. https://doi.org/10.3390/v14122817
- Short K.R., Kuiken T., Van Riel D. Role of endothelial cells in the pathogenesis of influenza in humans. J. Infect. Dis. 2019; 220(11): 1859–60. https://doi.org/10.1093/infdis/jiz349
- Armstrong S.M., Darwish I., Lee W.L. Endothelial activation and dysfunction in the pathogenesis of influenza A virus infection. Virulence. 2013; 4(6): 537–42. https://doi.org/10.4161/viru.25779
- Marchenko V.A., Barashkova S.V., Zelinskaya I.A., Toropova Ya.G., Ramsay E.S., Zhilinskaya I.N. Modulation of endothelial factors activity in human endothelial cells in influenza A(H1N1)PDM09 virus infection. Voprosy virusologii. 2021; 66(3): 198–210. https://doi.org/10.36233/0507-4088-48 https://elibrary.ru/wsxlvb (in Russian)
- Aird W.C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ. Res. 2007; 100(2): 158–73. https://doi.org/10.1161/01.RES.0000255691.76142.4a
- Zhang J., Defelice A.F., Hanig J.P., Colatsky T. Biomarkers of endothelial cell activation serve as potential surrogate markers for drug-induced vascular injury. Toxicol. Pathol. 2010; 38(6): 856–71. https://doi.org/10.1177/0192623310378866
- Matrosovich M.N., Matrosovich T.Y., Gray T., Roberts N.A., Klenk H.D. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc. Natl. Acad. Sci. USA. 2004; 101(13): 4620–4. https://doi.org/10.1073/pnas.0308001101
- Ibricevic A., Pekosz A., Walter M.J., Newby C., Battaile J.T., Brown E.G., et al. Influenza virus receptor specificity and cell tropism in mouse and human airway epithelial cells. J. Virol. 2006; 80(15): 7469–80. https://doi.org/10.1128/JVI.02677-05
- Abe Y., Smith C.W., Katkin J.P., Thurmon L.M., Xu X., Mendoza L.H., et al. Endothelial alpha 2,6-linked sialic acid inhibits VCAM-1-dependent adhesion under flow conditions. J. Immunol. 1999; 163(5): 2867–76.
- Cioffi D.L., Pandey S., Alvare D.F., Cioffi E.A. Terminal sialic acids are an important determinant of pulmonary endothelial barrier integrity. Am. J. Physiol. Lung Cell Mol. Physiol. 2012; 302(10): L1067–77. https://doi.org/10.1152/ajplung.00190.2011
- Denney L., Ho L.P. The role of respiratory epithelium in host defence against influenza virus infection. Biomed. J. 2018; 41(4): 218–33. https://doi.org/10.1016/j.bj.2018.08.004
- Herold S., Becker C., Ridge K.M., Budinger G.R. Influenza virus-induced lung injury: pathogenesis and implications for treatment. Eur. Respir. J. 2015; 45(5): 1463–78. https://doi.org/10.1183/09031936.00186214
- Herold S., Steinmueller M., von Wulffen W., Cakarova L., Pinto R., Pleschka S., et al. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J. Exp. Med. 2008; 205(13): 3065–77. https://doi.org/10.1084/jem.20080201
- Zeng H., Goldsmith C.S., Maines T.R., Belser J.A., Gustin K.M., Pekosz A., et al. Tropism and infectivity of influenza virus, including highly pathogenic avian H5N1 virus, in ferret tracheal differentiated primary epithelial cell cultures. J. Virol. 2013; 87(5): 2597–607. https://doi.org/10.1128/JVI.02885-12
- Kumlin U., Olofsson S., Dimock K., Arnberg N. Sialic acid tissue distribution and influenza virus tropism. Influenza Other Respir. Viruses. 2008; 2(5): 147–54. https://doi.org/10.1111/j.1750-2659.2008.00051.x
- Sugiyama M.G., Gamage A., Zyla R., Armstrong S.M., Advani S., Advani A., et al. Influenza virus infection induces platelet-endothelial adhesion which contributes to lung injury. J. Virol. 2015; 90(4): 1812–23. https://doi.org/10.1128/JVI.02599-15
- Lee S., Hirohama M., Noguchi M., Nagata K., Kawaguchi A. Influenza A virus infection triggers pyroptosis and apoptosis of respiratory epithelial cells through the type I interferon signaling pathway in a mutually exclusive manner. J. Virol. 2018; 92(14): e00396-18. https://doi.org/10.1128/JVI.00396-18
- Chan M.C., Chan R.W., Yu W.C., Ho C.C., Chui W.H., Lo C.K., et al. Influenza H5N1 virus infection of polarized human alveolar epithelial cells and lung microvascular endothelial cells. Respir. Res. 2009; 10(1): 102. https://doi.org/10.1186/1465-9921-10-102
- Zeng H., Pappas C., Belser J.A., Houser K.V., Zhong W., Wadford D.A., et al. Human pulmonary microvascular endothelial cells support productive replication of highly pathogenic avian influenza viruses: possible involvement in the pathogenesis of human H5N1 virus infection. J. Virol. 2012; 86(2): 667–78. https://doi.org/10.1128/JVI.06348-11
- Chan L.L.Y., Hui K.P.Y., Kuok D.I.T., Bui C.H.T., Ng K.C., Mok C.K.P., et al. Risk assessment of the tropism and pathogenesis of the highly pathogenic avian influenza A/H7N9 virus using ex vivo and in vitro cultures of human respiratory tract. J. Infect. Dis. 2019; 220(4): 578–88. https://doi.org/10.1093/infdis/jiz165
- Simon P., de La Vega M.A., Paradis É., Mendoza E., Coombs K.M., Kobasa D., et al. Avian influenza viruses that cause highly virulent infections in humans exhibit distinct replicative properties in contrast to human H1N1 viruses. Sci. Rep. 2016; 6: 24154. https://doi.org/10.1038/srep24154
- Han T., Lai Y., Jiang Y., Liu X., Li D. Influenza A virus infects pulmonary microvascular endothelial cells leading to microvascular leakage and release of pro-inflammatory cytokines. PeerJ. 2021; 9: e11892. https://doi.org/10.7717/peerj.11892
- Gu Y., Zuo X., Zhang S., Ouyang Z., Jiang S., Wang F., et al. The mechanism behind influenza virus cytokine storm. Viruses. 2021; 13(7): 1362. https://doi.org/10.3390/v13071362
- Tang B.M., Cootes T., McLean A.S. From influenza-induced acute lung injury to multiorgan failure. In: Annual Update in Intensive Care and Emergency Medicine 2019. 2018: 449–58. https://doi.org/10.1007/978-3-030-06067-1_35
- Yang Y., Tang H. Aberrant coagulation causes a hyper-inflammatory response in severe influenza pneumonia. Cell. Mol. Immunol. 2016; 13(4): 432–42. https://doi.org/10.1038/cmi.2016.1
- Zhang J. Biomarkers of endothelial activation and dysfunction in cardiovascular diseases. Rev. Cardiovasc. Med. 2022; 23(2): 73. https://doi.org/10.31083/j.rcm2302073
- Immanuel J., Yun S. Vascular inflammatory diseases and endothelial phenotypes. Cells. 2023; 12(12): 1640. https://doi.org/10.3390/cells12121640
- Mel’nikova Yu.S., Makarova T.P. Endothelial dysfunction as the key link of chronic diseases pathogenesis. Kazanskii meditsinskii zhurnal. 2015; 96(4): 659–65. https://doi.org/10.17750/KMJ2015-659 https://elibrary.ru/ubegwv (in Russian)
- Vlasova T.I., Petrishchev N.N., Vlasov T.D. Endothelial dysfunction as the typical pathological state. Regionarnoe krovoobrashchenie i mikrotsirkulyatsiya. 2022; 21(2): 4–15. https://doi.org/10.24884/1682-6655-2022-21-2-4-15 https://elibrary.ru/zheshs (in Russian)
- Yang Y., Bazhin A.V., Werner J., Karakhanova S. Reactive oxygen species in the immune system. Int. Rev. Immunol. 2013; 32(3): 249–70. https://doi.org/10.3109/08830185.2012.755176
- Bach F.H., Robson S.C., Ferran C., Winkler H., Millan M.T., Stuhlmeier K.M., et al. Endothelial cell activation and thromboregulation during xenograft rejection. Immunol. Rev. 1994; 141: 5–30. https://doi.org/10.1111/j.1600-065x.1994.tb00870.x
- Pober J.S., Sessa W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007; 7(10): 803–15. https://doi.org/10.1038/nri2171
- Bigildeev A.E., Chepurnykh Yu.F., Petinati N.A., Drize N.J. Features of the expression of NF-kB pathway genes in tissues of irradiated mice and in old animals. Radiatsionnaya biologiya. Radioekologiya. 2019; 59(6): 565–74. https://doi.org/10.1134/S0869803119060031 https://elibrary.ru/ebdunp (in Russian)
- Waitkus M.S., Harris D.P., DiCorleto P.E. Mechanisms of Endothelial Activation. In: Mackay I.R., Rose N.R., Diamond B., Davidson A., eds. Encyclopedia of Medical Immunology. New York: Springer; 2014. https://doi.org/10.1007/978-0-387-84828-0_183
- Endemann D.H., Schiffrin E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004; 15(8): 1983–92. https://doi.org/10.1097/01.ASN.0000132474.50966.DA
- Hadi H.A., Carr C.S., Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005; 1(3): 183–98.
- Widmer R.J., Lerman A. Endothelial dysfunction and cardiovascular disease. Glob. Cardiol. Sci. Pract. 2014; 2014(3): 291–308. https://doi.org/10.5339/gcsp.2014.43
- Han T., Lai Y., Jiang Y., Liu X., Li D. Influenza A virus infects pulmonary microvascular endothelial cells leading to microvascular leakage and release of pro-inflammatory cytokines. PeerJ. 2021; 9: e11892. https://doi.org/10.7717/peerj.11892
- Siragusa M., Thole J., Bibli S.I., Luck B., Loot A.E., de Silva K., et al. Nitric oxide maintains endothelial redox homeostasis through PKM2 inhibition. EMBO J. 2019; 38(17): e100938. https://doi.org/10.15252/embj.2018100938
- Marchenko V.A., Zelinskaya I.A., Toropova YA.G., Mukhametdinova D.V., Galagudza M.M., Lioznov D.A., et al. Duration of systemic alteration in vasomotor function of microvascular endothelium caused by the influenza A(H1N1)pdm09 virus. Regionarnoe krovoobrashchenie i mikrotsirkulyatsiya. 2023; 22(4): 74–86. https://doi.org/10.24884/1682-6655-2023-22-4-74-86 https://elibrary.ru/mmwnsf (in Russian)
- Boytsov SA. Influenza, novel coronavirus infection and cardiovascular diseases. Russian Kardiologicheskii vestnik. 2021; 16(1): 5–9. https://doi.org/10.17116/Cardiobulletin2021160115 https://elibrary.ru/zgvxkg (in Russian)
- Radi R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA. 2018; 115(23): 5839–48. https://doi.org/10.1073/pnas.1804932115
- Beckman J.S., Koppenol W.H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 1996; 271(5 Pt. 1): C1424–37. https://doi.org/10.1152/ajpcell.1996.271.5.C1424.
- Babizhayev M.A., Deyev A.I. Management of the virulent influenza virus infection by oral formulation of nonhydrolized carnosine and isopeptide of carnosine attenuating proinflammatory cytokine-induced nitric oxide production. Am. J. Ther. 2012; 19(1): e25–47. https://doi.org/10.1097/MJT.0b013e3181dcf589
- Vasina L.V., Petrishchev N.N., Vlasov T.D. Markers of endothelial dysfunction. Regionarnoe krovoobrashchenie i mikrotsirkulyatsiya. 2017; 16(1): 4–15. https://doi.org/10.24884/1682-6655-2017-16-1-4-15 https://elibrary.ru/yocujf (in Russian)
- Viemann D., Schmolke M., Lueken A., Boergeling Y., Friesenhagen J., Wittkowski H., et al. H5N1 virus activates signaling pathways in human endothelial cells resulting in a specific imbalanced inflammatory response. J. Immunol. 2011; 186(1): 164–73. https://doi.org/10.4049/jimmunol.0904170
- Teijaro J.R., Walsh K.B., Cahalan S., Fremgen D.M., Roberts E., Scott F., et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011; 146(6): 980–91. https://doi.org/10.1016/j.cell.2011.08.015
- Yu J., Sun X., Goie J.Y.G., Zhang Y. Regulation of host immune responses against influenza A virus infection by Mitogen-Activated Protein Kinases (MAPKs). Microorganisms. 2020; 8(7): 1067. https://doi.org/10.3390/microorganisms8071067
- Fontijn R.D., Volger O.L., van der Pouw-Kraan T.C., Doddaballapur A., Leyen T., Baggen J.M., et al. Expression of nitric oxide-transporting aquaporin-1 is controlled by KLF2 and marks non-activated endothelium in vivo. PLoS One. 2015; 10(12): e0145777. https://doi.org/10.1371/journal.pone.0145777
- Parmar K.M., Larman H.B., Dai G., Zhang Y., Wang E.T., Moorthy S.N., et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J. Clin. Invest. 2006; 116(1): 49–58. https://doi.org/10.1172/jci24787
- SenBanerjee S., Lin Z., Atkins G.B., Greif D.M., Rao R.M., Kumar A., et al. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J. Exp. Med. 2004; 199: 1305–15. https://doi.org/10.1084/jem.20031132
- Turpaev K.T. Transcription factor KLF2 and its role in the regulation of inflammatory processes. Biochemistry (Mosc.). 2020; 85(1): 54–67. https://doi.org/10.1134/S0006297920010058
- Azarenok A.A., Eropkina E.M., Prochukhanova A.R., Shaldzhyan A.A., Kozlova N.M., Kozeletskaya K.N., et al. The influenza viruses and their surface proteins impact on the metabolism of human blood vessel endothelium cells. Voprosy virusologii. 2013; 58(3): 25–7. https://elibrary.ru/pzxtur (in Russian)
- Hiyoshi M., Indalao I.L., Yano M., Yamane K., Takahashi E., Kido H. Influenza A virus infection of vascular endothelial cells induces GSK-3β-mediated β-catenin degradation in adherens junctions, with a resultant increase in membrane permeability. Arch. Virol. 2015; 160(1): 225–34. https://doi.org/10.1007/s00705-014-2270-5
- Betteridge K.B., Arkill K.P., Neal C.R., Harper S.J., Foster R.R., Satchell S.C., et al. Sialic acids regulate microvessel permeability, revealed by novel in vivo studies of endothelial glycocalyx structure and function. J. Physiol. 2017; 595(15): 5015–35. https://doi.org/10.1113/JP274167
- Taghavi S., Abdullah S., Shaheen F., Mueller L., Gagen B., Duchesne J., et al. Glycocalyx degradation and the endotheliopathy of viral infection. PLoS One. 2022; 17(10): e0276232. https://doi.org/10.1371/journal.pone.0276232
- Simionescu M. Structural biochemical and functional differentiation of the vascular endothelium. In: Risau W., ed. Morphogenesis of the Endothelium. Amsterdam: Harwood Academic; 2000: 1–21.
- Armstrong S.M., Wang C., Tigdi J., Si X., Dumpit C., Charles S., et al. Influenza infects lung microvascular endothelium leading to microvascular leak: role of apoptosis and claudin-5. PLoS One. 2012; 7(10): e47323 https://doi.org/10.1371/journal.pone.0047323
- Yang Y., Schmidt E.P. The endothelial glycocalyx: an important regulator of the pulmonary vascular barrier. Tissue Barriers. 2013; 1(1): e23494. https://doi.org/10.4161/tisb.23494
- Ferro T., Neumann P., Gertzberg N., Clements R., Johnson A. Protein kinase C-alpha mediates endothelial barrier dysfunction induced by TNF-alpha. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000; 278(6): L1107–17. https://doi.org/10.1152/ajplung.2000.278.6.L1107.
- Kim K., Jung H., Shin I., Choi B., Kim D. Induction of interleukin-1 beta (IL-1 β) is a critical component of lung inflammation during influenza A (H1N1) virus infection. J. Med. Virol. 2015; 87: 1104–12. https://doi.org/10.1002/jmv.24138.
- Wang S., Le T.Q., Kurihara N., Chida J., Cisse Y., Yano M., et al. Influenza virus-cytokine-protease cycle in the pathogenesis of vascular hyperpermeability in severe influenza. J. Infect. Dis. 2010; 202(7): 991–1001. https://doi.org/10.1086/656044
- Collins T., Read M.A., Neish A.S., Whitley M.Z., Thanos D., Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995; 9(10): 899-909.
- Guan X., Yang W., Sun X., Wang L., Ma B., Li H., et al. Association of influenza virus infection and inflammatory cytokines with acute myocardial infarction. Inflamm. Res. 2012; 61(6): 591–8. https://doi.org/10.1007/s00011-012-0449-3
- Singh V., Kaur R., Kumari P., Pasricha C., Singh R. ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and cardiovascular disorders. Clin. Chim. Acta. 2023; 548: 117487. https://doi.org/10.1016/j.cca.2023.117487
- George S.T., Lai J., Ma J., Stacey H.D., Miller M.S., Mullarkey C.E. Neutrophils and influenza: a thin line between helpful and harmful. Vaccines (Basel). 2021; 9(6): 597. https://doi.org/10.3390/vaccines9060597
- Tang B.M., Shojaei M., Teoh S., Meyers A., Ho J., Ball T.B., et al. Neutrophils-related host factors associated with severe disease and fatality in patients with influenza infection. Nat. Commun. 2019; 10(1): 3422. https://doi.org/10.1038/s41467-019-11249-y
- Narasaraju T., Yang E., Samy R.P., Ng H.H., Poh W.P., Liew A.A., et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 2011; 179(1): 199–210. https://doi.org/10.1016/j.ajpath.2011.03.013
- Saffarzadeh M., Juenemann C., Queisser M.A., Lochnit G., Barreto G., Galuska S.P., et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012; 7(2): e32366; http://doi.org/10.1371/journal.pone.0032366
- Schleef R.R., Bevilacqua M.P., Sawdey M., Gimbrone M.A. Jr., Loskutoff D.J. Cytokine activation of vascular endothelium. Effects on tissue-type plasminogen activator and type 1 plasminogen activator inhibitor. J. Biol. Chem. 1988; 263(12): 5797–803.
- Marchenko V., Mukhametdinova D., Amosova I., Lioznov D., Zhilinskaya I. Influenza A(H1N1)pdm09 virus alters expression of endothelial factors in pulmonary vascular endothelium in rats. Viruses. 2022; 14(11): 2518. https://doi.org/10.3390/v14112518
- Bussolino F., Camussi G., Baglioni C. Synthesis and release of platelet-activating factor by human vascular endothelial cells treated with tumor necrosis factor or interleukin 1 alpha. J. Biol. Chem. 1988; 263(24): 11856–61.
- Schastlivtsev I.V., Lobastov K.V., Tsaplin S.N., Mkrtychev D.S. Modern view on hemostasis system: cell theory. Meditsinskii sovet. 2019; (16): 72–7. https://doi.org/10.21518/2079-701X-2019-16-72-77 https://elibrary.ru/smgyfk (in Russian)
- Visseren F.L., Bouwman J.J., Bouter K.P., Diepersloot R.J., de Groot P.H., Erkelens D.W. Procoagulant activity of endothelial cells after infection with respiratory viruses. Thromb. Haemost. 2000; 84(2): 319–24.
- Zelaya H., Tada A., Vizoso-Pinto M.G., Salva S., Kanmani P., Agüero G., et al. Nasal priming with immunobiotic Lactobacillus rhamnosus modulates inflammation-coagulation interactions and reduces influenza virus-associated pulmonary damage. Inflamm. Res. 2015; 64(8): 589–602. https://doi.org/10.1007/s00011-015-0837-6
- Cesari M., Pahor M., Incalzi R.A. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc. Ther. 2010; 28(5): e72–91. https://doi.org/10.1111/j.1755-5922.2010.00171.x
- Slukhanchuk E.V., Bitsadze V.O., Khizroeva J.KH., Solopova A.G., Tsibizova V.I., Yakubova F., et al. The role of platelets in antiviral immunity. Akusherstvo, ginekologiya i reproduktsiya. 2022; 16(2): 204–12. https://doi.org/10.17749/2313-7347/ob.gyn.rep.2022.305 https://elibrary.ru/twhjna (in Russian)
- Iba T., Levi M., Thachil J., Levy J.H. Disseminated intravascular coagulation: the past, present, and future considerations. Semin. Thromb. Hemost. 2022; 48(8): 978–87. https://doi.org/10.1055/s-0042-1756300
- Jansen A.J.G., Spaan T., Low H.Z., Di Iorio D., van den Brand J., Tieke M., et al. Influenza-induced thrombocytopenia is dependent on the subtype and sialoglycan receptor and increases with virus pathogenicity. Blood Adv. 2020; 4(13): 2967–78. https://doi.org/10.1182/bloodadvances.2020001640
- Panina I.Yu., Rumyantsev A.Sh., Menshutina M.A., Achkasova V.V., Degtereva O.A., Tugusheva F.A., et al. Specific functions of the endothelium in chronic kidney disease. literature review and personal data. Nefrologiya. 2007; 11(4): 28–46. https://elibrary.ru/jtygjh (in Russian)
- Kim K.S., Jung H., Shin I.K., Choi B.R., Kim D.H. Induction of interleukin-1 beta (IL-1β) is a critical component of lung inflammation during influenza A (H1N1) virus infection. J. Med. Virol. 2015; 87(7): 1104–12. https://doi.org/10.1002/jmv.24138
- Choreño-Parra J.A., Jiménez-Álvarez L.A., Cruz-Lagunas A., Rodríguez-Reyna T.S., Ramírez-Martínez G., Sandoval-Vega M., et al. Clinical and immunological factors that distinguish COVID-19 from pandemic influenza A(H1N1). Front. Immunol. 2021; 12: 593595. https://doi.org/10.3389/fimmu.2021.593595
- Sumikoshi M., Hashimoto K., Kawasaki Y., Sakuma H., Suzutani T., Suzuki H., et al. Human influenza virus infection and apoptosis induction in human vascular endothelial cells. J. Med. Virol. 2008; 80(6): 1072–8. https://doi.org/10.1002/jmv.21185
- Cassina A.M., Hodara R., Souza J.M., Thomson L., Castro L., Ischiropoulos H., et al. Cytochrome c nitration by peroxynitrite. J. Biol. Chem. 2000; 275(28): 21409–15. https://doi.org/10.1074/jbc.M909978199
- Halder U.C., Bagchi P., Chattopadhyay S., Dutta D., Chawla-Sarkar M. Cell death regulation during influenza A virus infection by matrix (M1) protein: a model of viral control over the cellular survival pathway. Cell Death Dis. 2011; 2(9): e197. https://doi.org/10.1038/cddis.2011.75
- Winn R.K., Harlan J.M. The role of endothelial cell apoptosis in inflammatory and immune diseases. J. Thromb. Haemost. 2005; 3(8): 1815–24. https://doi.org/10.1111/j.1538-7836.2005.01378.x
- Shevchenko YU.L., Stojko YU.M., Gudymovich V.G. Endothelium as a target of pathological effects of viral infection. Vestnik Natsional’nogo mediko-khirurgicheskogo tsentra im. N.I. Pirogova. 2022; 17(2): 11–6. https://doi.org/10.25881/20728255_2022_17_2_11 https://elibrary.ru/yzfzkv (in Russian)
- Gui R, Chen Q. Molecular events involved in influenza A virus-induced cell death. Front. Microbiol. 2022; 12: 797789. https://doi.org/10.3389/fmicb.2021.797789
- Zhirnov O.P., Konakova T.E., Wolff T., Klenk H.D. NS1 protein of influenza A virus down-regulates apoptosis. J. Virol. 2002; 76(4): 1617–25. https://doi.org/10.1128/jvi.76.4.1617-1625.2002
- Stasakova J., Ferko B., Kittel C., Sereinig S., Romanova J., Katinger H., et al. Influenza A mutant viruses with altered NS1 protein function provoke caspase-1 activation in primary human macrophages, resulting in fast apoptosis and release of high levels of interleukins 1beta and 18. J. Gen. Virol. 2005; 86(Pt. 1): 185–95. https://doi.org/10.1099/vir.0.80422-0
- Wang X., Zheng T., Lin L., Zhang Y., Peng X., Yan Y., et al. Influenza A virus induces autophagy by its hemagglutinin binding to cell surface heat shock protein 90AA1. Front. Microbiol. 2020; 11: 566348. https://doi.org/10.3389/fmicb.2020.566348
- Othumpangat S., Noti J.D., McMillen C.M., Beezhold D.H. ICAM-1 regulates the survival of influenza virus in lung epithelial cells during the early stages of infection. Virology. 2016; 487: 85–94. https://doi.org/10.1016/j.virol.2015.10.005
- Tinoco R., Deiro M., Lin M., Bradley L. P-selectin regulation of T cell immunity during influenza virus infection (49.14). J. Immunol. 2011; 186(1 Suppl.): 49.14. https://doi.org/10.4049/jimmunol.186.Supp.49.14
- Short K.R., Veldhuis Kroeze E.J., Reperant L.A., Richard M., Kuiken T. Influenza virus and endothelial cells: a species specific relationship. Front. Microbiol. 2014; 5: 653. https://doi.org/10.3389/fmicb.2014.00653
- Garcia C.C., Russo R.C., Guabiraba R., Fagundes C.T., Polidoro R.B., Tavares L.P., et al. Platelet-activating factor receptor plays a role in lung injury and death caused by Influenza A in mice. PLoS Pathog. 2010; 6(11): e1001171. https://doi.org/10.1371/journal.ppat.1001171
- Morichi S., Morishita N., Takeshita M., Ishida Y., Oana S., Yamanaka G., et al. Vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) levels in the cerebrospinal fluid of children with influenza-associated encephalopathy. J. Infect. Chemother. 2017; 23(2): 80–4. https://doi.org/10.1016/j.jiac.2016.10.007
- Schmitz N., Kurrer M., Bachmann M.F., Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J. Virol. 2005; 79(10): 6441–8. https://doi.org/10.1128/JVI.79.10.6441-6448.2005
- Bawazeer A.O., Rosli S., Harpur C.M., Docherty C.A., Mansell A., Tate M.D. Interleukin-1β exacerbates disease and is a potential therapeutic target to reduce pulmonary inflammation during severe influenza A virus infection. Immunol. Cell Biol. 2021; 99(7): 737–48. https://doi.org/10.1111/imcb.12459
- Zhilinskaya I.N., Marchenko V.A., Kharchenko E.P. Comparison of fragments in human hemostatic proteins that mimics fragments in proteins of A/H1N1 viruses and coronaviruses. Molekulyarnaya genetika, mikrobiologiya i virusologiya. 2022; 40(4): 43–6. https://doi.org/10.17116/molgen20224004143 https://elibrary.ru/mwqoig (in Russian)
- Goldsteyn E.M. Influenza-associated mortality for circulatory and respiratory causes during the 2013-2014 through the 2018-2019 influenza seasons in Russia. Mezhdunarodnyi zhurnal prikladnykh i fundamental’nykh issledovanii. 2019; (12-1): 9–16. https://doi.org/10.17513/mjpfi.12945 https://elibrary.ru/dhthqt (in Russian)
Supplementary files