Synthesis and characterization of human rotavirus A (Reoviridae: Sedoreovirinae: Rotavirus: Rotavirus A) virus-like particles
- Authors: Cherepushkin S.A.1, Tsibezov V.V.1, Yuzhakov A.G.1, Latyshev O.E.1, Alekseev K.P.1, Altayeva E.G.2, Khametova K.M.1, Vorkunova G.K.1, Yuzhakova K.A.1, Grebennikova T.V.1
-
Affiliations:
- FSBI National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
- Vetbiochem, LLC
- Issue: Vol 66, No 1 (2021)
- Pages: 55-64
- Section: ORIGINAL RESEARCHES
- URL: https://ogarev-online.ru/0507-4088/article/view/118173
- DOI: https://doi.org/10.36233/0507-4088-27
- ID: 118173
Cite item
Full Text
Abstract
Introduction. Rotavirus infection is the leading cause of acute gastroenteritis among infants. The development of new vaccines against rotavirus A is urgent because the virus has many genotypes, some of which have regional prevalence. Virus-like particles (VLP) is a promising way to create effective and safe vaccine preparations.
The purpose of the study is to develop the technology for the production of VLP, containing VP2, VP4, VP6 and VP7 of viral genotypes prevalent on the territory of the Russian Federation, and to give its molecular genetic and virological characteristics.
Material and methods. The virulent strain Wa G1P[8] of human RV A adapted to MARC-145 cell culture has been used. It was cultured and purified according to the method described by the authors earlier. Standard molecular genetic and cytological methods were used: gene synthesis; cloning into transfer plasmids; recombinant baculoviruses production in Bac-to-Bac expression system; VLP production in the insect cells; centrifugation in sucrose solution; enzyme-linked immunosorbent assay (ELISA); electron microscopy (EM); polyacrylamide gel electrophoresis (SDS-PAGE) and western blot analysis.
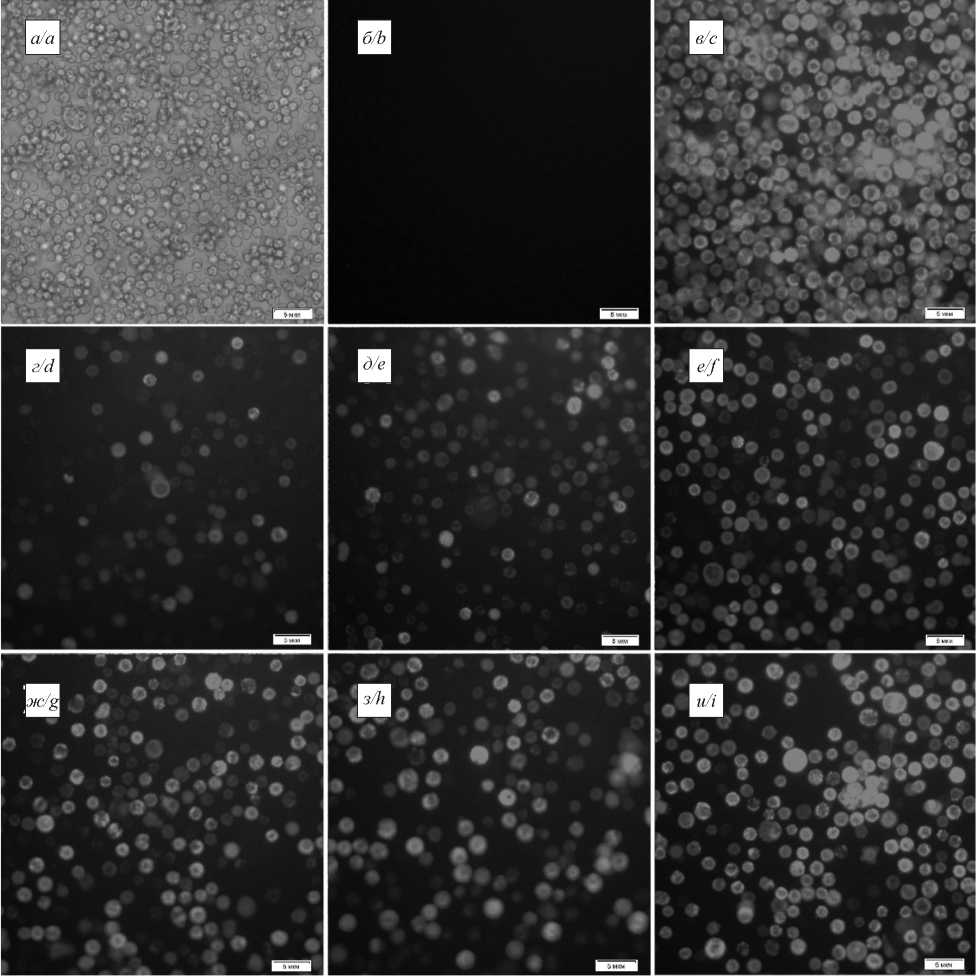
Results. VP4 and VP7 of the six most represented in Russia genotypes: G1, G2, G4, G9, P4, P8, as well as VP2 and VP6 were selected for VLP production. Recombinant baculoviruses were obtained with codon frequencies optimized for insect cells. Cabbage loopper (Trichoplusia ni) cell culture was coinfected with different combinations of baculoviruses, and VLP consisting of 2-4 proteins were produced. VLP were purified by centrifugation. The size and morphology of the particles matched the rotavirus A virion (by EM). The presence of rotavirus A proteins in VLP was confirmed by the ELISA, SDS-PAGE and western blot analysis.
Conclusion. The technology for the synthesis of three-layer VLP consisting of VP2, VP4, VP6 and VP7 has been developed and optimized. The resulting VLP composition represents 6 serotypes of VP4 and VP7, which are most represented on the territory of Russia, and can be used for vaccine development.
Full Text
##article.viewOnOriginalSite##About the authors
S. A. Cherepushkin
FSBI National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Author for correspondence.
Email: cherepushkin1@gmail.com
ORCID iD: 0000-0002-1734-5369
Stanislav A. Cherepushkin - Researcher, Laboratory of Applied Virology and Biotechnology.
123098, Moscow
Russian FederationV. V. Tsibezov
FSBI National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: tsibezov@yandex.ru
ORCID iD: 0000-0003-2150-5764
Valeriy V. Tsibezov - Ph.D. (Biol.), Leading Researcher of the Laboratory of Specific Prophylaxis.
123098, Moscow
Russian FederationA. G. Yuzhakov
FSBI National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: anton_oskol@mail.ru
ORCID iD: 0000-0002-0426-9678
Anton G. Yuzhakov - Ph.D. (Biol.), Senior Researcher of the Laboratory of Molecular Diagnostics.
123098, Moscow
Russian FederationO. E. Latyshev
FSBI National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: oleglat80@mail.ru
ORCID iD: 0000-0003-1934-9635
Oleg E. Latyshev - Ph.D. (Biol.), Senior Researcher of the Laboratory of Molecular Diagnostics.
123098, Moscow
Russian FederationK. P. Alekseev
FSBI National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: kalekseev@hotmail.com
ORCID iD: 0000-0001-9536-3127
Konstantin P. Alekseev - Ph.D. (Biol.), Senior Researcher of the Laboratory of Applied Virology and Biotechnology.
123098, Moscow
Russian FederationE. G. Altayeva
Vetbiochem, LLC
Email: e.altaeva@gmail.com
ORCID iD: 0000-0001-7188-1704
Erzhena G. Altaeva - Ph.D. (Biol.), Senior Process Engineer.
Vetbiochem, LLC
Russian FederationK. M. Khametova
FSBI National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: kizkhalum@yandex.ru
ORCID iD: 0000-0002-8461-600X
Kizkhalum M. Khametova - Ph.D. (Biol.), Researcher of the Laboratory of Specific Prophylaxis.
123098, Moscow
Russian FederationG. K. Vorkunova
FSBI National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: chekh-ks@mail.ru
ORCID iD: 0000-0003-3130-5029
Galina K. Vorkunova - Ph.D., D.Sci (Biol.), Leading Researcher of the Laboratory of Molecular Diagnostics.
123098, Moscow
Russian FederationK. A. Yuzhakova
FSBI National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: g.k.vorkunova@gmail.com
ORCID iD: 0000-0003-1346-3744
Ksenia A. Yuzhakova - Junior Researcher of the Laboratory of Molecular Diagnostics.
123098, Moscow
Russian FederationT. V. Grebennikova
FSBI National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: t_grebennikova@mail.ru
ORCID iD: 0000-0002-6141-9361
Tatyana V. Grebennikova - Ph.D., D.Sci (Biol.), Prof., Corresponding Member of RAS, Head of the Laboratory of Molecular Diagnostics.
123098, Moscow
Russian FederationReferences
- Crawford S.E., Ramani S., Tate J.E., Parashar U.D., Svensson L., Hagbom M., et al. Rotavirus infection HHS Public Access. JAMA Pediatrics. 2018; 172(Suppl. 3): 50-3. https://doi.org/10.1038/nrdp.2017.83.Rotavirus.
- Государственный доклад «О состоянии санитарно-эпидемиологического благополучия населения в Российской Федерации в 2018 году». Москва; 2019. Available at: https://www.rospotrebnadzor.ru/upload/iblock/798/gosudarstvennyy-doklad-o-sostoyanii-sanitarno_epidemiologicheskogo-blagopoluchiya-naseleniya-v-rossiyskoy-fed-eratsii-v-2018-godu.pdf (accessed January 28, 2021).
- Государственный доклад «О состоянии санитарно-эпидемиологического благополучия населения в Российской Федерации в 2019 году». Москва; 2020. Available at: https://www.rospotreb-nadzor.ru/upload/iblock/8e4/gosdoklad-za-2019_seb_29_05.pdf (accessed January 28, 2021).
- Burke R.M., Tate J.E., Kirkwood C.D., Steele A.D., Parashar U.D. Current and new rotavirus vaccines. Curr. Opin. Infect. Dis. 2019; 32(5): 435-44. https://doi.org/10.1097/QC0.0000000000000572.
- Wang Y., Li J., Liu P., Zhu F. The performance of licensed rotavirus vaccines and the development of a new generation of rotavirus vaccines: a review. Hum. Vaccin. Immunother. 2020; 1-17. https://doi.org/10.1080/21645515.2020.1801071.
- Yen C., Healy K., Tate J.E., Parashar U.D., Bines J., Neuzil K., et al. Rotavirus vaccination and intussusception - Science, surveillance, and safety: A review of evidence and recommendations for future research priorities in low- and middle-income countries. Hum. Vac-cin. Immunother. 2016; 12(10): 2580-9. https://doi.org/10.1080/21645515.2016.1197452.
- Parez N. Rotavirus gastroenteritis: why to back up the development of new vaccines? Comp. Immunol. Microbiol. Infect. Dis. 2008; 31(2-3): 253-69. https://doi.org/10.1016/j.cimid.2007.07.005.
- Crawford S.E., Labbe M., Cohen J., Burroughs M.H., Zhou Y.J., Estes M.K. Characterization of virus-like particles produced by the expression of rotavirus capsid proteins in insect cells. J. Virol. 1994; 68(9): 5945-52. https://doi.org/10.1128/jvi.68.9.5945-5952.1994.
- Azevedo M.P., Vlasova A.N., Saif L.J. Human rotavirus virus-like particle vaccines evaluated in a neonatal gnotobiotic pig model of human rotavirus disease. Expert Rev. Vaccines. 2013; 12(2): 16981. https://doi.org/10.1586/erv.13.3.
- Changotra H., Vij A. Rotavirus virus-like particles (RV-VLPs) vaccines: An update. Rev. Med. Virol. 2017; 27(6). https://doi.org/10.1002/rmv.1954.
- Doro R., Laszlo B., Martella V., Leshem E., Gentsch J., Parashar U., et al. Review of global rotavirus strain prevalence data from six years post vaccine licensure surveillance: Is there evidence of strain selection from vaccine pressure? Infect. Genet. Evol. 2014; 28: 446-61. https://doi.org/10.1016/j.meegid.2014.08.017.
- Matthijnssens J., Ciarlet M., Rahman M., Attoui H., Estes M.K., Gentsch J.R., et al. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch. Virol. 2008; 153(8): 1621-9. https://doi.org/10.1007/s00705-008-0155-1.
- Matthijnssens J., Bilcke J., Ciarlet M., Martella V, Banyai K., Rahman M., et al. Rotavirus disease and vaccination: impact on genotype diversity. Future Microbiol. 2009; 4(10): 1303-16. https://doi.org/10.2217/fmb.09.96.
- Хаметова К.М., Алексеев К.П., Южаков А.Г., Костина Л.В., Раев С.А., Мусиенко М.И., и др. Молекулярно-биологические свойства клонированного штамма Wa ротавируса А человека. Вопросы вирусологии. 2019; 64(1): 16-22. https://doi.org/10.18821/0507-4088-2019-64-1-16-22.
- Латышев О.Е., Елисеева О.В., Костина Л.В., Алексеев К.П., Хаметова К.М., Алтаева Е.Г., и др. Оценка иммуногенной активности клонированного штамма WA ротавируса А человека. Вопросы вирусологии. 2019; 64(4): 156-64. https://doi.org/10.36233/0507-4088-2019-64-4-156-164.
- Kumar А., Charpilienne А., Cohen J. Nucleotide sequence of the gene encoding for the RNA binding protein (VP2) of RF bovine rotavirus. Nucleic Acids Res. 1989; 17(5): 2126. https://doi.org/10.1093/nar/17.5.2126.
- Charpilienne A., Nejmeddine M., Berois M., Parez N., Neumann E., Hewat E., et al. Individual rotavirus-like particles containing 120 molecules of fluorescent protein are visible in living cells. J. Biol. Chem. 2001; 276(31): 29361-7. https://doi.org/10.1074/jbc.M101935200.
- Ivashechkin A.A., YuzhakovA.G., Grebennikova T.V., Yuzhakova K.A., Kulikova N.Y., Kisteneva L.B., et al. Genetic diversity of group A rotaviruses in Moscow in 2018-2019. Arch. Virol. 2020; 165(3): 691-702. https://doi.org/10.1007/s00705-020-04534-5.
- Kiseleva V., Faizuloev E., Meskina E, Marova A., Oksanich A., Samart-seva T., et al. Molecular-genetic characterization of human rotavirus A strains circulating in Moscow, Russia (2009-2014). Virol. Sin. 2018; 33(4): 304-13. https://doi.org/10.1007/s12250-018-0043-0.
- Jere K.C., O’Neill H.G., Potgieter A.C., Van Dijk A.A. Chimaeric virus-like particles derived from consensus genome sequences of human rotavirus strains co-circulating in Africa. PLoS One. 2014; 9(9): e105167. https://doi.org/10.1371/journal.pone.0105167.
- Donaldson B., Lateef Z., Walker G.F., Young S.L., Ward V.K. Virus-like particle vaccines: immunology and formulation for clinical translation. Expert Rev. Vaccines. 2018; 17(9): 833-49. https://doi.org/10.1080/14760584.2018.1516552.
- Estes M.K., Crawford S.E., Penaranda M.E., Petrie B.L., Burns J.W., Chan W.K., et al. Synthesis and immunogenicity of the rotavirus major capsid antigen using a baculovirus expression system. J. Virol. 1987; 61(5): 1488-94. https://doi.org/10.1128/jvi.61.5.1488-1494.1987.
- Labbe M., Charpilienne A., Crawford S.E., Estes M.K., Cohen J. Expression of rotavirus VP2 produces empty corelike particles. J. Virol. 1991; 65(6): 2946-52. https://doi.org/10.1128/jvi.65.6.2946-2952.1991.
- Vieira H.L.A., Estevao C., Roldao A., Peixoto C.C., Sousa M.F.Q., Cruz P.E., et al. Triple layered rotavirus VLP production: Kinetics of vector replication, mRNA stability and recombinant protein production. J. Biotechnol. 2005; 120(1): 72-82. https://doi.org/10.1016/j.jbiotec.2005.03.026.
- Vicente T., Sousa M.F.Q., Peixoto C., Alves P.M., Mota J.P., Car-rondo M.J.T. Anion-exchange membrane chromatography for purification of rotavirus-like particles. J. Memb. Sci. 2008; 311(1-2): 270-83. https://doi.org/10.1016/j.memsci.2007.12.021.
- Kim Y., Chang K., Kim W., Saif L.J. Production of hybrid double-or triple-layered virus-like particles of group A and C rotaviruses using a baculovirus expression system. Virology. 2002; 302(1): 1-8. https://doi.org/10.1006/viro.2002.1610.
- O’Neal C.M., Crawford S.E., Estes M.K., Conner M.E. Rotavirus virus-like particles administered mucosally induce protective immunity. J. Virol. 1997; 71(11): 8707-17. https://doi.org/10.1128/jvi.71.11.8707-8717.1997.
- Ciarlet M., Crawford S.E., Barone C., Bertolotti-Ciarlet A., Ramig R.F., Estes M.K., et al. Subunit rotavirus vaccine administered parenterally to rabbits induces active protective immunity. J. Virol. 1998; 72(11): 9233-46. https://doi.org/10.1128/jvi.72.11.9233-9246.1998.
- Gonzalez A.M., Azevedo M.S.P., Jung K., Vlasova A., Zhang W., Saif L.J. Innate immune responses to human rotavirus in the neonatal gnotobiotic piglet disease model. Immunology. 2010; 131(2): 242-56. https://doi.org/10.1111/j.1365-2567.2010.03298.x.
Supplementary files