Vaccination with virus-like particles containing hemagglutinin protects the lungs of mice with postifluenza bacterial pneumonia: virological, microbiological and clinical data
- Authors: Falynskova I.N.1, Egorov A.Y.1,2, Poddubikov A.V.1, Vartanova N.O.1, Kartashova N.P.1, Glubokova E.A.1, Mkhitarov V.A.3, Dzhalilova D.S.3, Makarova O.V.3, Leneva I.A.1
-
Affiliations:
- I.I. Mechnikov Research Institute of Vaccines and Sera
- Smorodintsev Research Institute of Influenza
- Research Institute of Human Morphology
- Issue: Vol 65, No 3 (2020)
- Pages: 150-158
- Section: ORIGINAL RESEARCHES
- URL: https://ogarev-online.ru/0507-4088/article/view/118137
- DOI: https://doi.org/10.36233/0507-4088-2020-65-3-150-158
- ID: 118137
Cite item
Full Text
Abstract
Introduction. Influenza is a severe viral disease, a frequent complication of which is a secondary bacterial pneumonia. Influenza vaccines prevent secondary bacterial complications. Virus-like particles are one of the promising areas for the development of new vaccines.
The aim of this work is to study the correlation of the pathomorphological characteristics of the lungs with clinical, virological, and microbiological markers of the disease at vaccination with virus-like particles (VLPs), containing hemagglutinin (HA) of influenza virus (HA-Gag-VLPs) in a murine model of secondary bacterial pneumonia induced by S. pneumoniae after influenza infection.
Material and methods. BALB/c mice were vaccinated with VLPs containing influenza HA. After 21 days, mice were infected with two strains of influenza viruses, homologous and non-homologous, and 5 days after viral infection, were infected with S. pneumoniae. The vaccination effect was evaluated by morphological, virological (titer of the virus in the lungs) and microbiological (titer of bacteria in the lungs) data, and was confirmed by clinical data (survival, change in body weight).
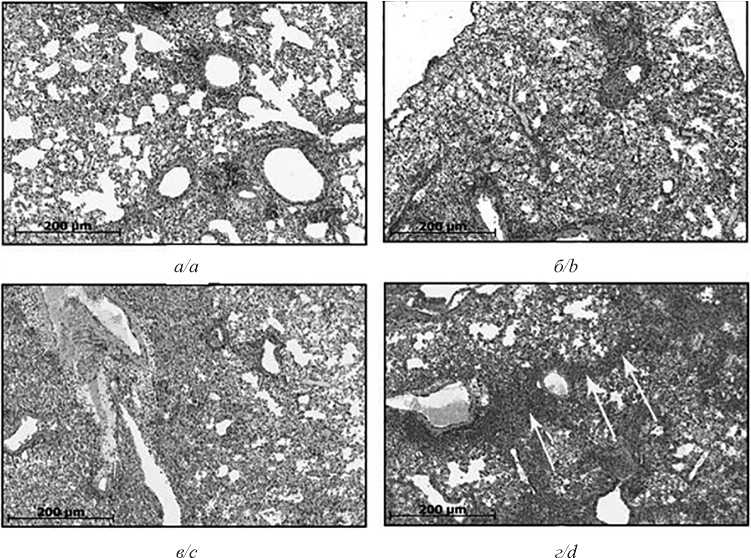
Results. Immunization with HA-Gag-VLPs, followed by infection with a homologous influenza virus and S. pneumoniae, reduced the area of foci of inflammation, inhibited the replication of the virus and bacteria in the lungs, and also protected animals from death and reduced their weight loss. Immunization with HA-Gag-VLPs upon infection with a heterologous strain and S. pneumoniae did not affect these criteria.
Conclusion. The immunization with HA-Gag-VLPs prevented the viral replication, providing a reduction of S. pneumoniae titer and the degree of lung damage, protecting animals from the disease in a murine model of secondary bacterial pneumonia, induced by S. pneumoniae, after influenza infection with homologous strain of the virus.
Full Text
##article.viewOnOriginalSite##About the authors
I. N. Falynskova
I.I. Mechnikov Research Institute of Vaccines and Sera
Author for correspondence.
Email: falynskova@mail.ru
ORCID iD: 0000-0001-9836-9620
Irina N. Falynskova, researcher of Laboratory experimental virology
Moscow, 105064
Russian FederationA. Yu. Egorov
I.I. Mechnikov Research Institute of Vaccines and Sera; Smorodintsev Research Institute of Influenza
ORCID iD: 0000-0003-2068-3745
Andrej Yu. Egorov
Moscow, 105064
Saint Petersburg, 197022
Russian FederationA. V. Poddubikov
I.I. Mechnikov Research Institute of Vaccines and Sera
ORCID iD: 0000-0001-8962-4765
Alexander V. Poddubikov
Moscow, 105064
Russian FederationN. O. Vartanova
I.I. Mechnikov Research Institute of Vaccines and Sera
ORCID iD: 0000-0002-6372-9910
Nune O. Vartanova
Moscow, 105064
Russian FederationN. P. Kartashova
I.I. Mechnikov Research Institute of Vaccines and Sera
ORCID iD: 0000-0003-2096-5080
Nadezhda P. Kartashova
Moscow, 105064
Russian FederationE. A. Glubokova
I.I. Mechnikov Research Institute of Vaccines and Sera
ORCID iD: 0000-0002-5925-9733
Ekaterina A. Glubokova
Moscow, 105064
Russian FederationV. A. Mkhitarov
Research Institute of Human Morphology
ORCID iD: 0000-0002-4427-1991
Vladimir A. Mkhitarov
Moscow, 117418
Russian FederationD. S. Dzhalilova
Research Institute of Human Morphology
ORCID iD: 0000-0002-1337-7160
Dzhuliya S. Dzhalilova
Moscow, 117418
Russian FederationO. V. Makarova
Research Institute of Human Morphology
ORCID iD: 0000-0001-8581-107X
Olga V. Makarova
Moscow, 117418
Russian FederationI. A. Leneva
I.I. Mechnikov Research Institute of Vaccines and Sera
ORCID iD: 0000-0002-7755-2714
Irina A. Leneva
Moscow, 105064
Russian FederationReferences
- Morens D.M., Taubenberger J.K., Fauci A.S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J. Infect. Dis. 2008; 198(7): 962-70. DOI: http://doi.org/10.1086/591708
- Klausberger M., Leneva I.A., Egorov A., Strobl F., Ghorbanpour S.M., Falynskova I.N., et al. Off-target effects of an insect cell-expressed influenza HA-pseudotyped gag-VLP preparation in limiting postinfluenza Staphylococcus Aureus infections. Vaccine. 2019; 38(4): 859-67. DOI: http://doi.org/10.1016/j.vaccine.2019.10.083
- Klausberger M., Leneva I.A., Falynskova I.N., Vasiliev K., Poddubikov A.V., Lindner C., et al. The potential of influenza HA-specific immunity in mitigating lethality of postinfluenza pneumococcal infections. Vaccines (Basel). 2019; 7(4): 187. DOI: http://doi.org/10.3390/vaccines7040187
- Chaussee M.S., Sandbulte H.R., Schuneman M.J., DePaula F.P., Addengast L.A., Schlenker E.H., et al. Inactivated and live, attenuated influenza vaccines protect mice against influenza: Streptococcus pyogenes super-infections. Vaccine. 2011; 29(21): 3773-81. DOI: http://doi.org/10.1016/j.vaccine.2011.03.031
- Okamoto S., Kawabata S., Fujitaka H., Uehira T., Okuno Y., Hamada S. Vaccination with formalin-inactivated influenza vaccine protects mice against lethal influenza Streptococcus pyogenes superinfection. Vaccine. 2004; 22(21-22): 2887-93. DOI: http://doi.org/10.1016/j.vaccine.2003.12.024
- Mina M., Klugman K., McCullers J. Live attenuated influenza vaccine, but not pneumococcal conjugate vaccine, protects against increased density and duration of pneumococcal carriage after influenza infection in pneumococcal colonized mice. J. Infect. Dis. 2013; 208(8): 1281-5. DOI: http://doi.org/10.1093/infdis/jit317
- Carrat F., Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine. 2007; 25(39-40): 6852-62. DOI: http://doi.org/10.1016/j.vaccine.2007.07.027
- Desheva Y., Leontieva G., Kramskaya T., Grabovskaya K.B., Karev V., Mamontov A., et al. Mucosal vaccine based on attenuated influenza virus and the group B Streptococcus recombinant peptides protectedmice from influenza and S. pneumoniae infections. PLoS One. 2019; 14(6): e0218544. DOI: http://doi.org/10.1371/journal.pone.0218544
- Ленёва И.А., Егоров А.Ю., Фалынскова И.Н., Махмудова Н.Р., Карташова Н.П., Глубокова Е.А. и др. Индукция вторичной бактериальной пневмонии у мышей при заражении пандемическим и лабораторными штаммами вируса гриппа H1N1. Журнал микробиологии, эпидемиологии и иммунобиологии. 2019; 96(2): 68- 74. DOI: http://doi.org/10.36233/0372-9311-2019-1-68-74
- Махмудова Н.Р., Ленёва И.А., Ларионова Н.В., Поддубиков А.В., Фалынскова И.Н., Карташова Н.П. и др. Безопасность аттенуированной и рекомбинантной интраназальных гриппозных вакцин в условиях развития вторичной бактериальной суперинфекции. Журнал микробиологии, эпидемиологии и иммунобиологии. 2019; 96(6): 30-9. DOI: http://doi.org/10.36233/0372-9311-2019-6-30-39
Supplementary files